Peripheral nerve stimulation for chronic pain
Peripheral neuropathic pain is highly prevalent but unfortunately incredibly difficult to treat. Current management algorithms include a trial-and-error attempts with available pharmacological options and nerve blocks.
For patients who have failed conservative therapy, neuromodulation can be considered such as spinal cord stimulators. Unfortunately, spinal cord stimulators (SCS) are an invasive technology that involves altering pain signals as they travel along spinal cord to the brain. SCS implantation requires a two-step procedure with initial placement of spinal stimulator leads attached to an external battery.
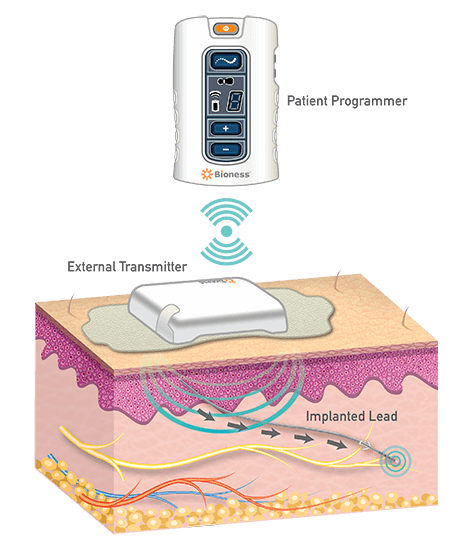
Advances in neuromodulation devices have allowed for a lead implantation without the need for an implantable pulse generator (i.e., internalized battery). Peripheral nerve stimulators (PNS) devices that are implanted for peripheral neuropathic pain. PNS devices are leads that can be implanted under ultrasound guidance.They can communicate wirelessly with an external pulse transmitter (EPT) using conductive radiofrequency signals. The EPT is positioned directly over the implanted lead by mounting it on a disposable electrode patch worn on the skin. Externalization of the PNS device allows for a less invasive and less expensive neuromodulation therapy.
Early clinical evidence has demonstrated that use of Peripheral Nerve Stimulation has resulted in significant pain relief (~75% reduction in pain scores).1 Further, transient (approximately 30 minutes) electrical nerve stimulation has reduced pain several hours after therapy, signifying a possible lasting relief of these devices.1 Patients with Peripheral Nerve Stimulation devices have also found significant improvements in function, work abilities, sleep, quality of life, and reduction in medication use.1,2
While using electrical current to provide analgesia has been used for decades, it was only until 2015 that peripheral nerve stimulators without an implantable generator became commercially available in the US; Health Canada only approved these devices in January 2018. Currently, Bioness is the only vendor available on the Canadian market.
StudioAthletica is one of few Canadian clinics with expertise in placing these Peripheral Nerve Stimulators. If you are interested to see if you are candidate, please have your physician send over a referral for an assessment.
References:
- Soin A, Syed Shah N, Fang ZP. High‐frequency electrical nerve block for postamputation pain: a pilot study. Neuromodulation: Technology at the Neural Interface. 2015 Apr;18(3):197-206.
- Deer T, Pope J, Benyamin R, Vallejo R, Friedman A, Caraway D, Staats P, Grigsby E, Porter McRoberts W, McJunkin T, Shubin R. Prospective, multicenter, randomized, double‐blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation: Technology at the Neural Interface. 2016 Jan;19(1):91-100.

